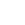Scientists explain emergence of especially dangerous pathogens of tuberculosis
In a new study, published in the journal Bioorganic Chemistry, scientists have explained why Mycobacterium tuberculosis survives in inappropriate environmental conditions inside the human body.
The results of this study may help to better understand the role of enzymes in bacteria in the development of resistance against the human immune system and drugs.
The authors of the study examined the crystal structure and function of rubredoxin B (RubB), a metalloprotein (a protein containing metal atoms) that ensures the proper functioning of cytochrome P450, another protein important for bacterial survival. Scientists have a hypothesis, according to which, tubercle bacilli have learned to switch rubredoxin into a mode that ensures the effective use of iron in conditions of iron deficiency, which is observed during the formation of granulomas (nodules) in the lungs.
As a result, it turned out that rubredoxin B is associated with heme monooxygenases, which are important for the metabolism of oxysterols (they play a signaling role in the maintenance of immunity) and the host's anti-tuberculosis drugs. In other words, M. tuberculosis has the ability to neutralize xenobiotics.
Previous research has shown that one of the cytochromes supported by RubB may act against SQ109, a promising potential drug for multidrug-resistant tuberculosis.
According to the WHO, every year around the world 10 million people fall ill with tuberculosis, and 1.5 million people die from it, which makes it the main infectious killer in the world. Particularly dangerous strains of the tubercle bacillus, which have appeared in recent decades, have multiple drug resistance, mainly antibiotics, which greatly complicates the treatment of the infection.
 How Not to Go Bankrupt on Black Friday: 5 Smart Shopping Tips
How Not to Go Bankrupt on Black Friday: 5 Smart Shopping Tips
 IDBank warmly hosted children from the "Music for Future" Foundation.
IDBank warmly hosted children from the "Music for Future" Foundation.
 Ucom’s 5G network launched in 11 new cities
Ucom’s 5G network launched in 11 new cities
 UPay has joined Idram's Open QR infrastructure
UPay has joined Idram's Open QR infrastructure
 A Free Mastercard and 10% idcoins with Cashless Payments. IDBank
A Free Mastercard and 10% idcoins with Cashless Payments. IDBank
 The Results of the 19th Annual International Microelectronics Olympiad Summarized in Yerevan
The Results of the 19th Annual International Microelectronics Olympiad Summarized in Yerevan
 AraratBank Named Best Investor Relations Bank Armenia 2024
AraratBank Named Best Investor Relations Bank Armenia 2024
 General Director of Ucom participated in Silicon Mountains technological summit
General Director of Ucom participated in Silicon Mountains technological summit
 Saving together: IDBank and Idram
Saving together: IDBank and Idram
 ARARATBANK AND SINGAPORE GFTN SIGN MEMORANDUM OF UNDERSTANDING FOR DIGITAL TRANSFORMATION
ARARATBANK AND SINGAPORE GFTN SIGN MEMORANDUM OF UNDERSTANDING FOR DIGITAL TRANSFORMATION
 Andron Participates in the Tomorrow Mobility World Congress 2024: Driving Innovation in E-Mobility
Andron Participates in the Tomorrow Mobility World Congress 2024: Driving Innovation in E-Mobility
 Acba bank and American Express Expand Collaboration in Armenia
Acba bank and American Express Expand Collaboration in Armenia
 The Silicon Mountains technology summit to be held with the support of Ucom
The Silicon Mountains technology summit to be held with the support of Ucom
 Evocabank became the first bank to join Idram’s open QR infrastructure
Evocabank became the first bank to join Idram’s open QR infrastructure
 25% Off on RIA Money Transfers to Ukraine at AraratBank
25% Off on RIA Money Transfers to Ukraine at AraratBank
 Yerevan to Host Unprecedented Serbian-Armenian Music Concert
Yerevan to Host Unprecedented Serbian-Armenian Music Concert
 AraratBank Stands with My Forest Armenia to establish Charles Aznavour Forest
AraratBank Stands with My Forest Armenia to establish Charles Aznavour Forest
 Ucom and Sunchild NGO install another solar plant in Areni
Ucom and Sunchild NGO install another solar plant in Areni
 4,401,021 AMD to COAF. The November beneficiary is “Armenia Tree Project”
4,401,021 AMD to COAF. The November beneficiary is “Armenia Tree Project”
 Go Digital or Go Home: Sergey Arakelyan
Go Digital or Go Home: Sergey Arakelyan
 Evocabank became the first bank to join Idram’s open QR infrastructure
Evocabank became the first bank to join Idram’s open QR infrastructure
 Junius: A Financial Literacy Game in the Idram Junior App
Junius: A Financial Literacy Game in the Idram Junior App
 Which Book to Choose as a Gift for the Book Giving Day? A Piece of Advice from Idram and IDBank
Which Book to Choose as a Gift for the Book Giving Day? A Piece of Advice from Idram and IDBank
 The Christmas gift from the Galaxy Group of Companies is the restoration of a song extricated by
Khrimian Hayrik
The Christmas gift from the Galaxy Group of Companies is the restoration of a song extricated by
Khrimian Hayrik
 Galaxy Group of Companies replaced the traditional New Year's gifts with houses of kindness and peace crafted by the chi...
Galaxy Group of Companies replaced the traditional New Year's gifts with houses of kindness and peace crafted by the chi...
 Ucom օffers combining mobile and fixed services in Unity packages and save up to 45%
Ucom օffers combining mobile and fixed services in Unity packages and save up to 45%
 I personally promised the greatest athlete that he will definitely witness the eternity of his own fame. Gagik Tsarukyan...
I personally promised the greatest athlete that he will definitely witness the eternity of his own fame. Gagik Tsarukyan...
 Donate blood, help to live
Donate blood, help to live